Defining a range of stimulation parameters for optical cochlear implants
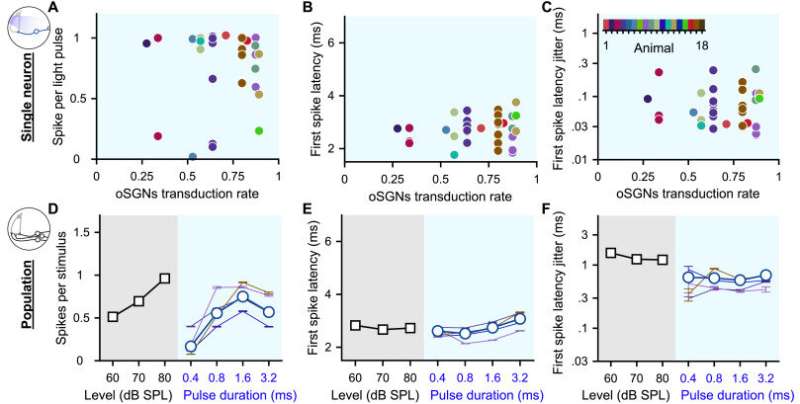
Heterogeneous optogenetic activation of the SGNs and artificial population response. A-C. Quantification of the spikes per light pulse (A), first spike latency (B) and first spike latency jitter (C) as a function of the average SGN transduction rate. Measurements were done in response to 100 pps light pulse trains of 1.6 ms (n = 35 SGNs). A color code was used per animal. No correlation was found (correlation coefficient test). D-F. Quantification of the number of spikes per stimulus (D), first spike latency (E) and first spike latency jitter (F) computed across SGNs recorded from a single animal (≥4 SGNs, thin colored line, same color code as in A-C) and all recorded SGNs (gray background, 100 cps acoustic click train: n = 20 SGNs with the best frequency within the 16 kHz octave band; blue background, optogenetics, 100 pps light pulse trais: n = 47 SGNs). Credit: Brain Stimulation (2023). DOI: 10.1016/j.brs.2023.01.1671
Optogenetics, the control of genetically modified cells with light, has revolutionized the life sciences and medicine. It allows the activity of cells and their networks to be controlled in a targeted manner via light pulses. This opens up completely new perspectives for the therapy of dysfunctions of sensory systems, such as hearing and vision.
The potential of optogenetic treatments for the functional recovery of sensory systems and their clinical feasibility have recently been demonstrated for the visual system. The optogenetic treatment of hearing loss and deafness by the optical cochlear implant (oCI) is still in the preclinical stage. Preclinical studies and simulations suggest that hearing with light has the potential to enable near-physiological auditory impression, including the recognition of emotional tones and complex melodies.
Dr. Antoine Tarquin Huet, Junior Fellow at the Göttingen Cluster of Excellence Multiscale Bioimaging: From Molecular Machines to Networks of Excitable Cells (MBExC), explores the requirements that must be met for the clinical use of the oCI in humans. “Hearing with light requires the oCI to convert acoustic signals into a pattern of light signals, which then stimulate the neurons in the cochlea in an appropriate manner. Optogenetic stimulation must be precisely tailored to the coding properties of the auditory nerve cells,” says Huet from the Institute for Auditory Neuroscience of the University Medical Center Göttingen (UMG).
“In the current study, we defined appropriate stimulation parameters within which the control of auditory neurons using future optogenetic prostheses, such as the oCI, light is plausible.” In their recently published study in the journal Brain Stimulation, the Göttingen hearing researchers describe how a fast and reliable characterization of future optogenetic tools can be achieved, which can then be used to study the processing of neuronal signals between the ear and the brain.
The restoration of hearing via an optogenetic approach allows to bypass dysfunctional or missing sensory cells by targeting the activity of downstream auditory neurons with light pulses. The approach requires a gene therapy in which the auditory nerve cells are made light-sensitive by inserting light-sensitive ion channels, so-called channelrhodopsins. The implantable optical stimulator of the oCI is used for targeted stimulation with light.
This leads to the opening of the channels and an influx of ions, an action potential occurs, and the nerve cell is electrically excited. Light pulses for stimulating the auditory nerve can be used much more precisely than electricity, thus enabling to activate much smaller areas with fewer auditory nerve cells than with the electrical CI used so far. The cellular specificity and spatial confinement of optogenetic stimulation promise to improve functional recovery far beyond what is possible with current electrical cochlear implants.
Research findings in detail
The prerequisite for restoring hearing with light is that the optical cochlear implant translates the incoming sound information into light signals, which in turn activate the nerve cells in the cochlea in a suitable manner. To pave the way for clinical trials and thus future use in humans, the first step is to define the boundaries within which the control of nerve cell activity in the cochlea can succeed.
“Optimal control of neuronal activity with light is far from trivial,” Huet said. “It requires good tuning of the production of the channelrhodopsins used and their cellular membrane incorporation, optimal matching of optogenetic stimulation parameters to the coding properties of the targeted neuron population, and the right choice of laser diodes.”
In their study, the Göttingen scientists present a parameter range for the optogenetic control of neurons and applied it to the auditory pathway, which requires high temporal accuracy of stimulation. They studied how light pulses of defined intensity and duration control the activation of individual auditory neurons in the cochlea of mice. To that end, they introduced Chronos, a naturally occurring channelrhodopsin that is able to respond extremely quickly to light stimulation, into the spiral ganglion neurons (SGN) of the mouse cochlea.
They demonstrated that by adjusting the duration of light pulses a graded activation of auditory neurons can be achieved. This is of interest for the optimal use of the laser diodes. Moreover, they defined the optimal duration as well as an upper limit for the frequency of light pulses under the given conditions.
Interestingly, the population of optogenetically controlled auditory neurons exhibited great diversity. “From a theoretical point of view, this functional diversity is a key factor that expands the amount of encoded information and increases its reliability,” Huet says.
In addition, the findings indicate that neurons downstream of the SGNs could be excited in a near-physiological manner when SGNs are optogenetically stimulated. The findings of the Göttingen hearing researchers will pave the way for designing the sound-coding strategies of future optical cochlear implants.